Essure Failure After 5 Years
With a total of 96 per 1 000 women ending up pregnant after hysteroscopic sterilization the effectiveness of essure after 10 years is apparently as low.
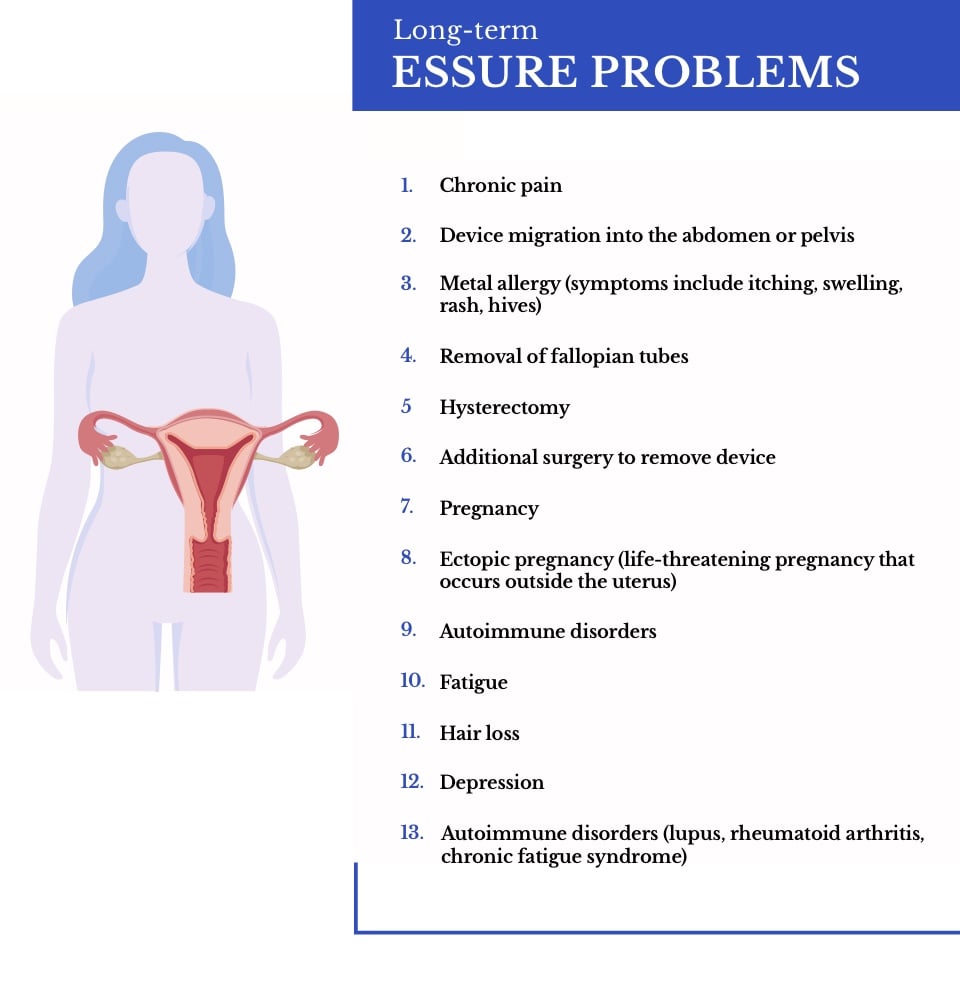
Essure failure after 5 years. Headaches nausea dizziness and vomiting may also occur. The company that made the essure system of birth control discontinued sales of the device in the u s. The fda continues to review the available information about essure and the experiences of patients who have had essure since the fda approved it in 2002. A 2015 review found the effectiveness of essure is unclear due to the low quality of evidence.
Later studies that followed up women after five years found that the risk of pregnancy over 10 years was 10 times greater than for those who chose the older laparoscopic sterilization method. There have been many reports of pregnancy in women who have had an essure device implanted. Some women experience pelvic or back discomfort. These essure side effects usually only last a few days according to bayer.
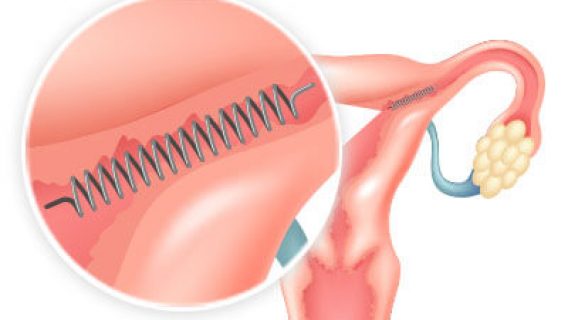
Data shows that after 5 years from the date a woman has essure placed successfully she has less than a 1 percent chance of getting pregnant. The actual certified numbers from the ten year study in 2012 looked at 500 000 women globally and determined an efficacy of 99 83 after a confirming hsg. Essure was designed as an implantable birth control device that permanently blocked the fallopian tubes in women. For example implant failure or.
According to a recent study however the rate of pregnancy after essure is about 5 7 much more than was initially reported by the birth control manufacturer. A computer model shows that 57 per 1 000 women would become pregnant within the first year of choosing essure for permanent birth control. All pregnancies carry some risk to women and their. With perfect use another review found evidence of a 99 8 effective based on 5 years of follow up.
Try 9 6 which is roughly 10 15 times the failure rate of tubal ligation the surgical procedure essure was designed to replace. It s not just the 0 5 that bayer would have us believe either. Sales of the device stopped in 2017 in all other countries.